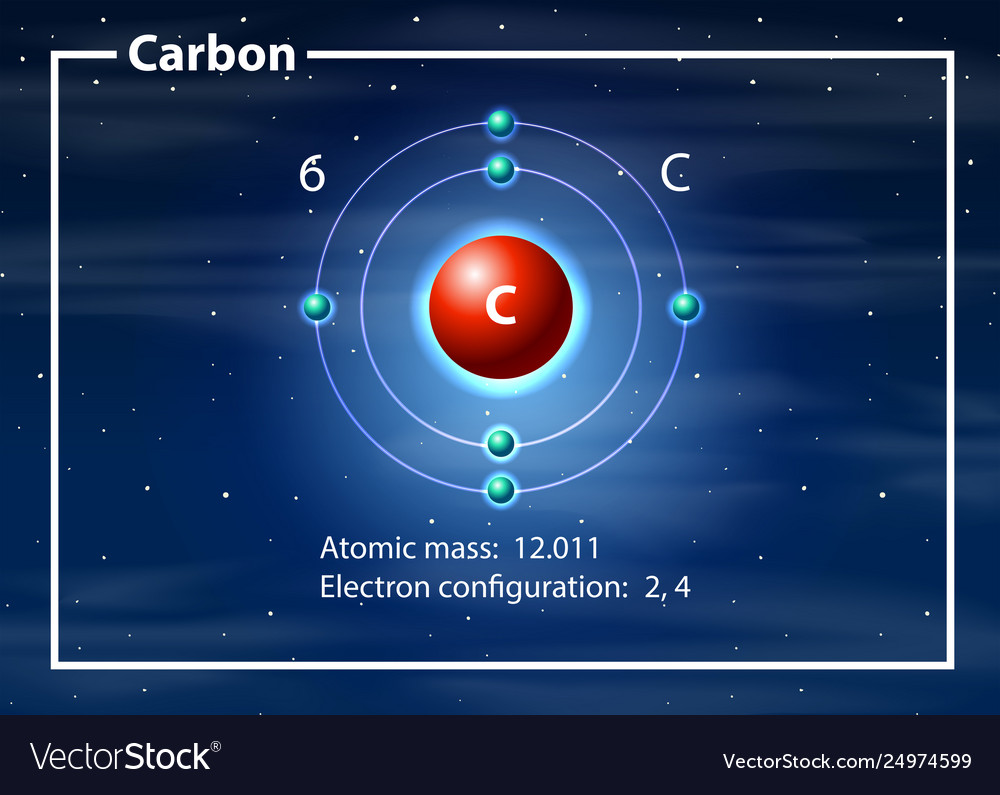
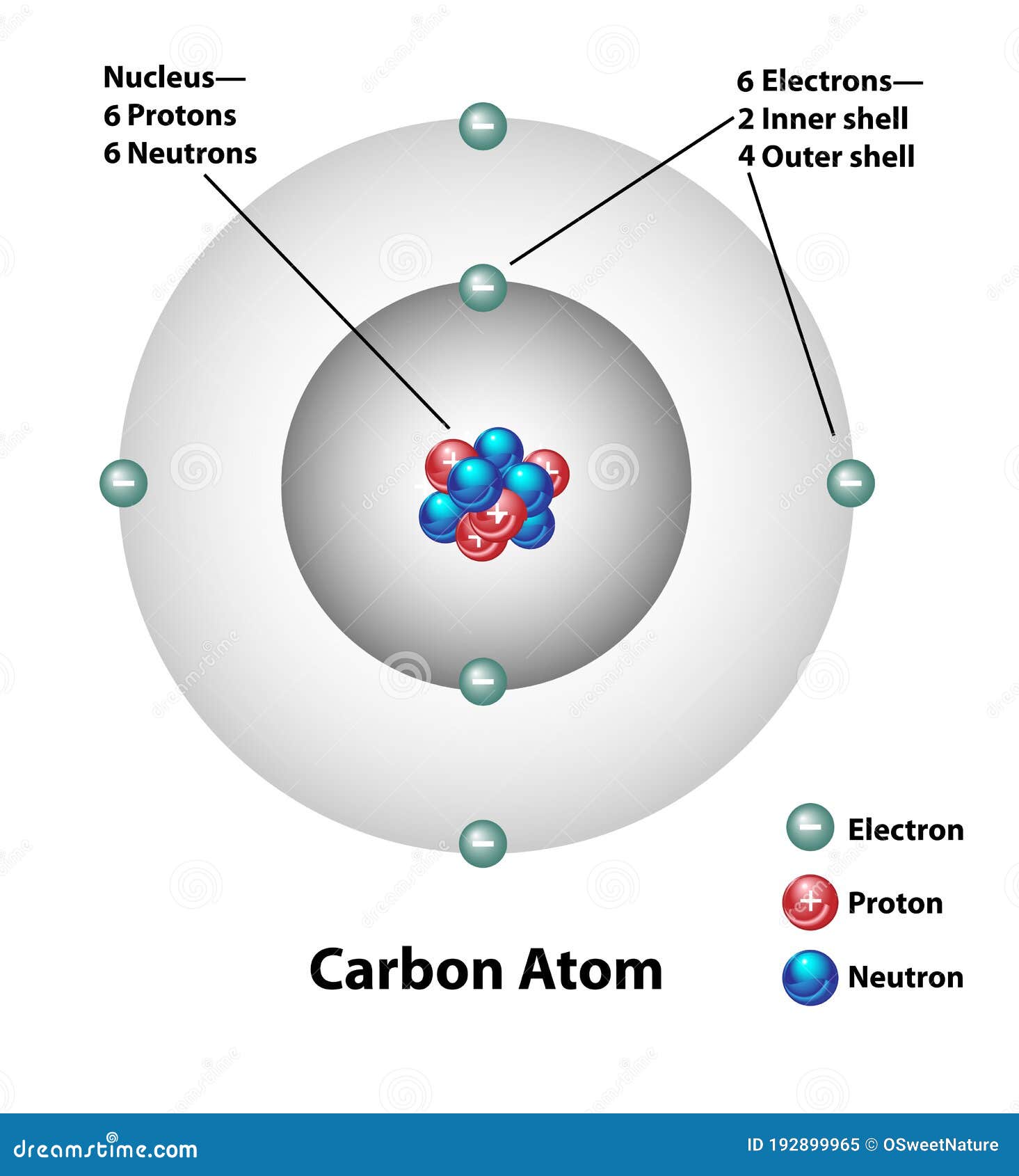
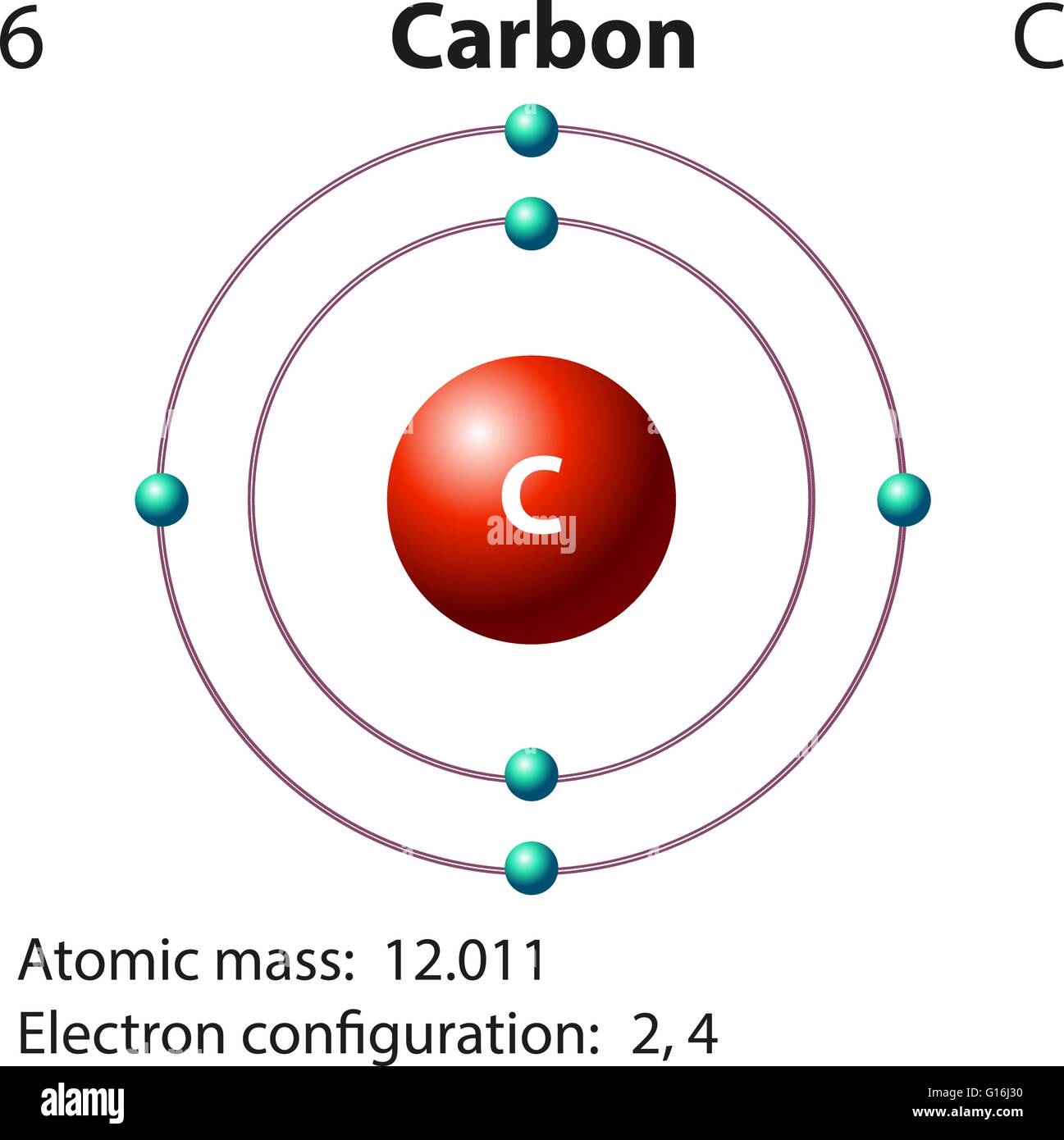
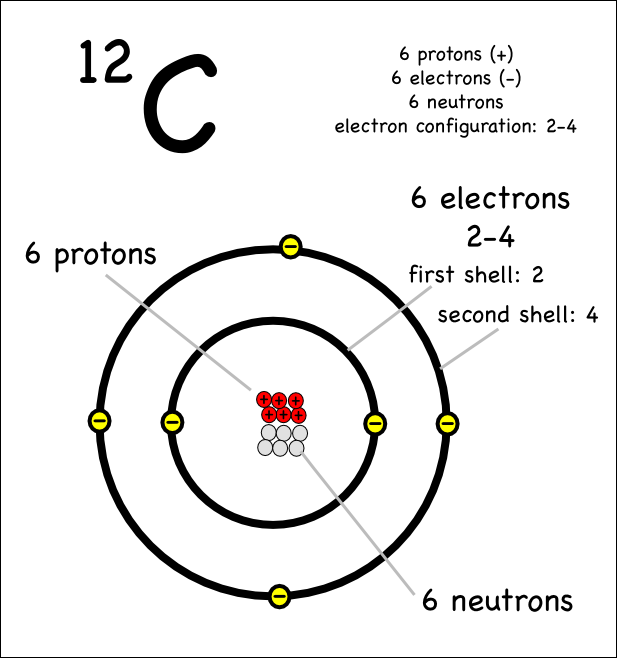
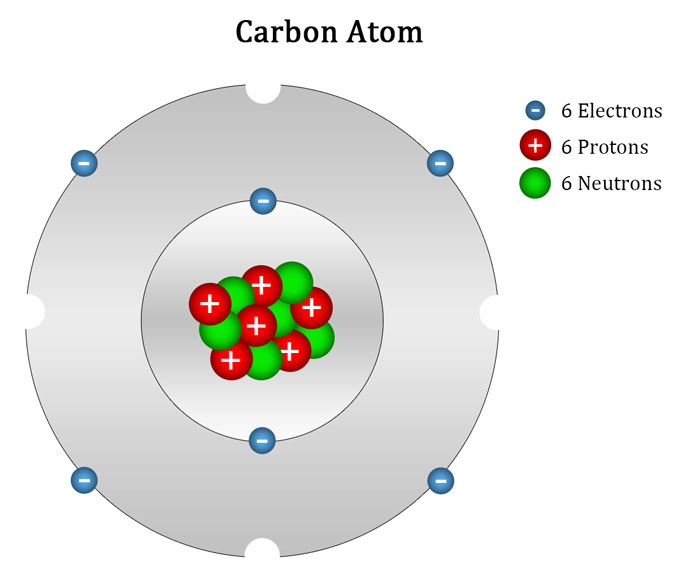
Web we will use this information to draw the bohr model of the carbon atom. As long as it’s carbon it has six protons. An atomic diagram of carbon provides a visual representation of the carbon atom, its structure, and its electrons. Web explain how electrons are shared between atoms. Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons.
In the first step, we will draw the nucleus of the carbon atom. Web the commonest way to draw structural formulae. An atomic diagram of carbon provides a visual representation of the carbon atom, its structure, and its electrons. As long as it’s carbon it has six protons. But they look different, and it turns out they are:
Web each contains 5 carbon atoms and 12 hydrogen atoms. Web the carbon atom is sp hybridized and oxygen atoms are sp2, making the overall molecule sp hybridized. Web atomic structure (bohr model) for magnesium (mg) carbon has 2 electrons in its first shell and 4 in its second shell. Carbon is a chemical element with the symbol c and atomic number 6, making it one of the essential building blocks of life on earth. An atomic diagram of carbon provides a visual representation of the carbon atom, its structure, and its electrons.
Hybridization can be understood by 2 methods, one by understanding the combination of the orbitals and 2nd by using a simple formula. As long as it’s carbon it has six protons. An atomic diagram of carbon provides a visual representation of the carbon atom, its structure, and its electrons. All structures must begin with an atom. Web this image of a carbon atom is taken from a postsecondary chemistry textbook. Carbon makes up about 0.025 percent of earth's crust. Two oxygen atoms are found at the terminals, where they share electrons and form a double bond with the carbon atom. Web in co2, the carbon atom is in the central position as it is the least electronegative atom in the molecule. But they look different, and it turns out they are: Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons. Web we will use this information to draw the bohr model of the carbon atom. And each oxygen is six, and we have one of them, so six. The properties of these substances are similar but not exactly the same. The substance to the left has a boiling point of 9.5 ºc, making it a gas at room temperature. Hydrogens that are attached to elements other than carbon are shown.
The Full Page Where It Appears Can Be Seen Below.
Hydrogens that are attached to elements other than carbon are shown. Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons. Web explain how electrons are shared between atoms. It has symbol c and atomic number 6.
Carbon Is A Chemical Element With The Symbol C And Atomic Number 6, Making It One Of The Essential Building Blocks Of Life On Earth.
There are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon up to 4. This atom is also balanced in charge so it also needs six electrons. Each hydrogen is one valence electron, but we have two of them, so 1 times 2. Create a chain of carbon atoms.
The Substance To The Left Has A Boiling Point Of 9.5 ºc, Making It A Gas At Room Temperature.
An atomic diagram of carbon provides a visual representation of the carbon atom, its structure, and its electrons. And each oxygen is six, and we have one of them, so six. Carbon makes up about 0.025 percent of earth's crust. Web carbon is the central atom in the lewis structure of co 2 because it is the least electronegative element in the molecule.
For This, We Will First Have To Calculate The Number Of Protons And Neutrons Present In This Atom.
Web the carbon atom is sp hybridized and oxygen atoms are sp2, making the overall molecule sp hybridized. Web if you want (or need) to draw a model of an atom, we'll show you how! Later on in this chapter and throughout this book we will see examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom bears a positive or negative formal charge, respectively. You can use the element to create new atoms or modify existing atoms.