Na cro bez product hino, → product 2 h,so → product 3 febry h, so draw product 1 draw product 2 on oh x x draw product draw product 2 on oh ŏ o incorre draw product 3 oh š incorrect Show the formal charges, where applicable. Draw the organic product of each step in the following synthesis. Show the formal charges, where applicable. When it reacts with an alkene, it breaks the double bond and forms two alcohol groups.
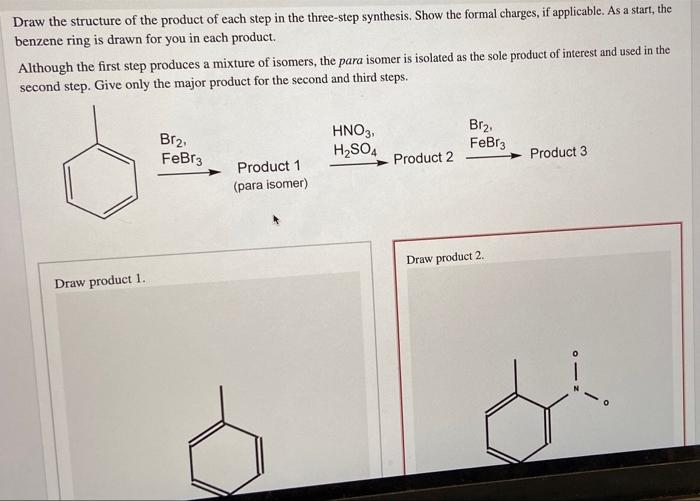
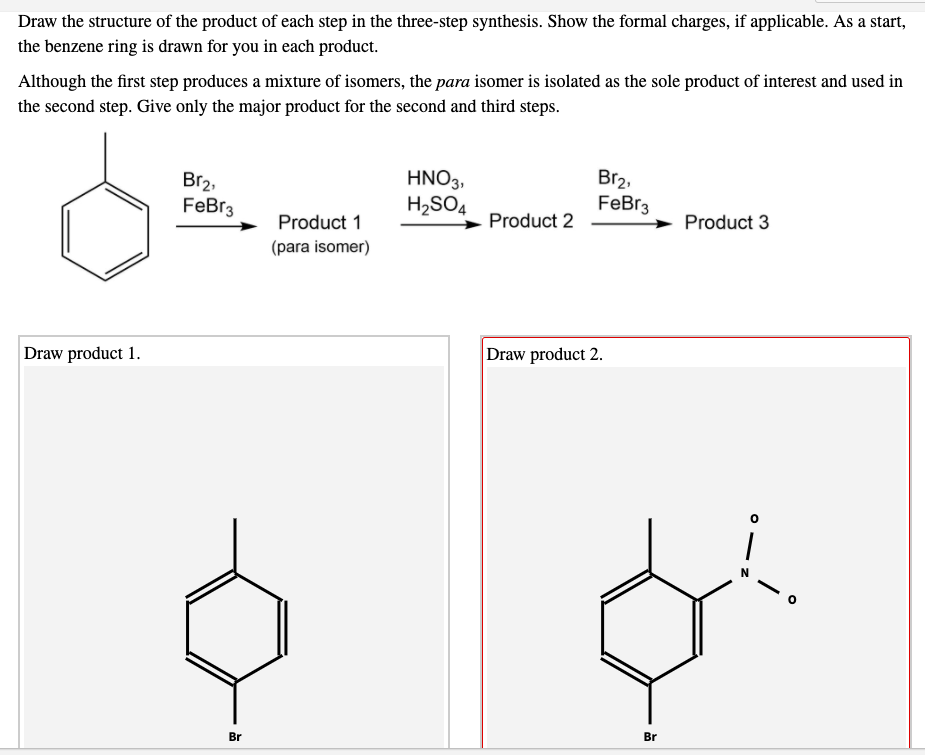
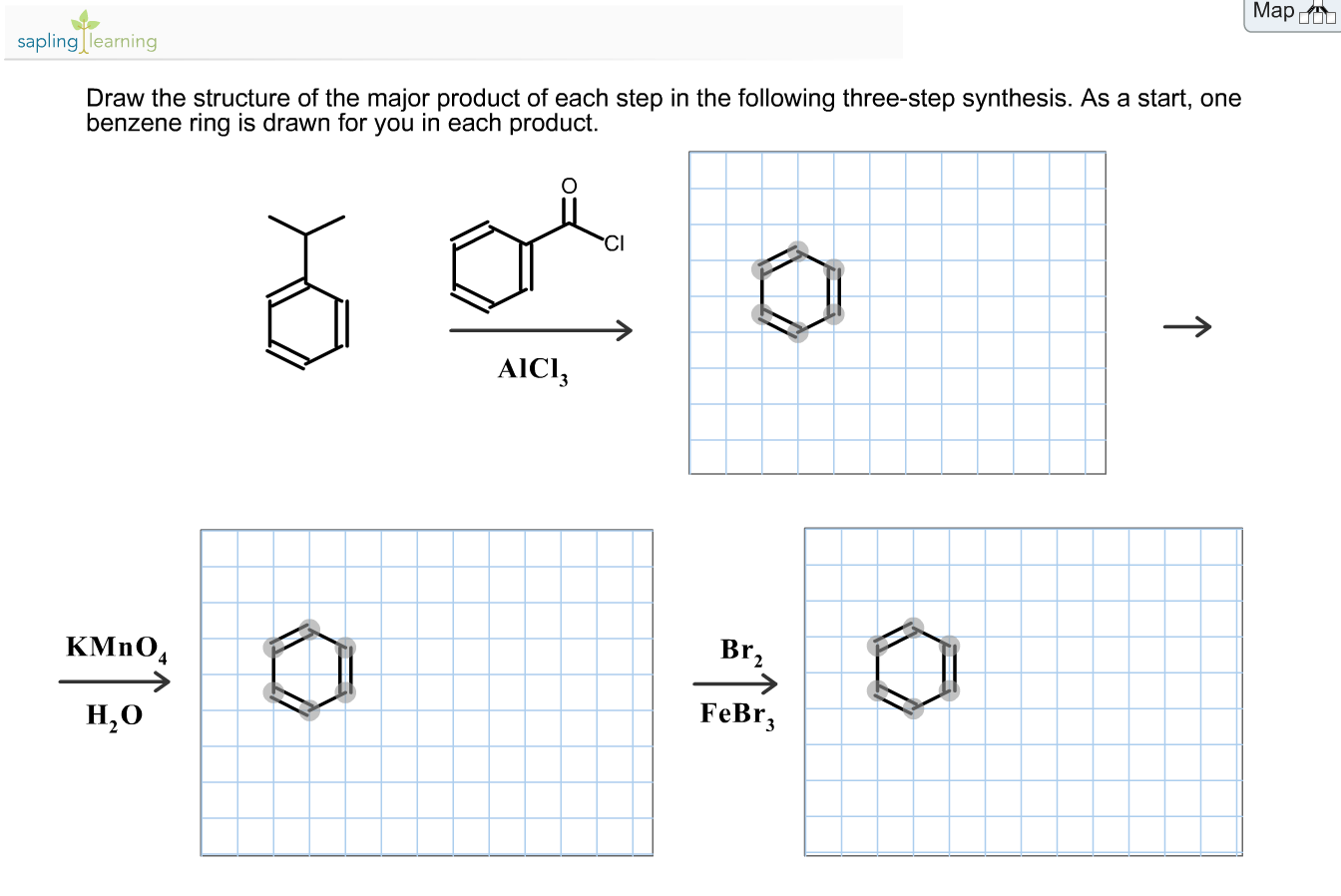
Show the formal charges, where applicable. As a start, the benzene ring is drawn for you in each product. As a start, the benzene ring is drawn for you in each product 1.kmno, naoh,δ hno 2.h30 h2so, febr 02 previous ⓧ give up & view solution check answer next exit hint the first step is an oxidation. Draw the organic product of each step in the following synthesis. So, the product 1 will have two alcohol groups on the carbon atoms where the double bond was present.
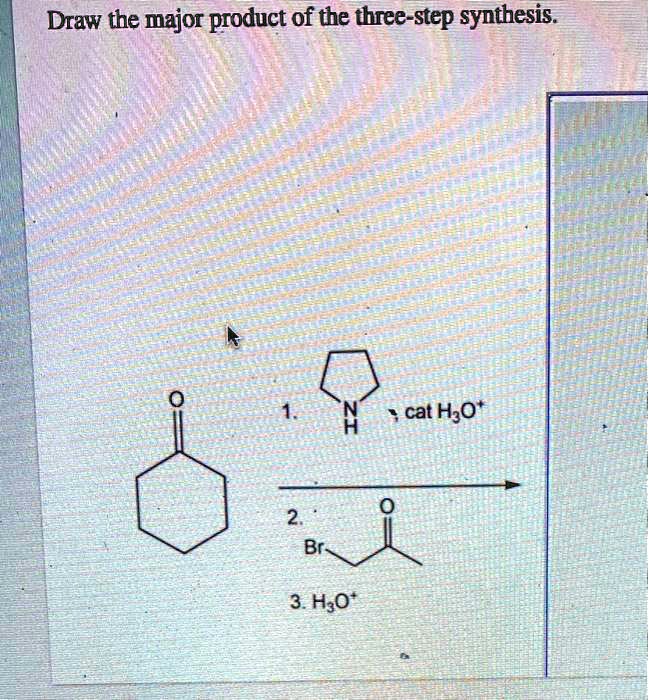
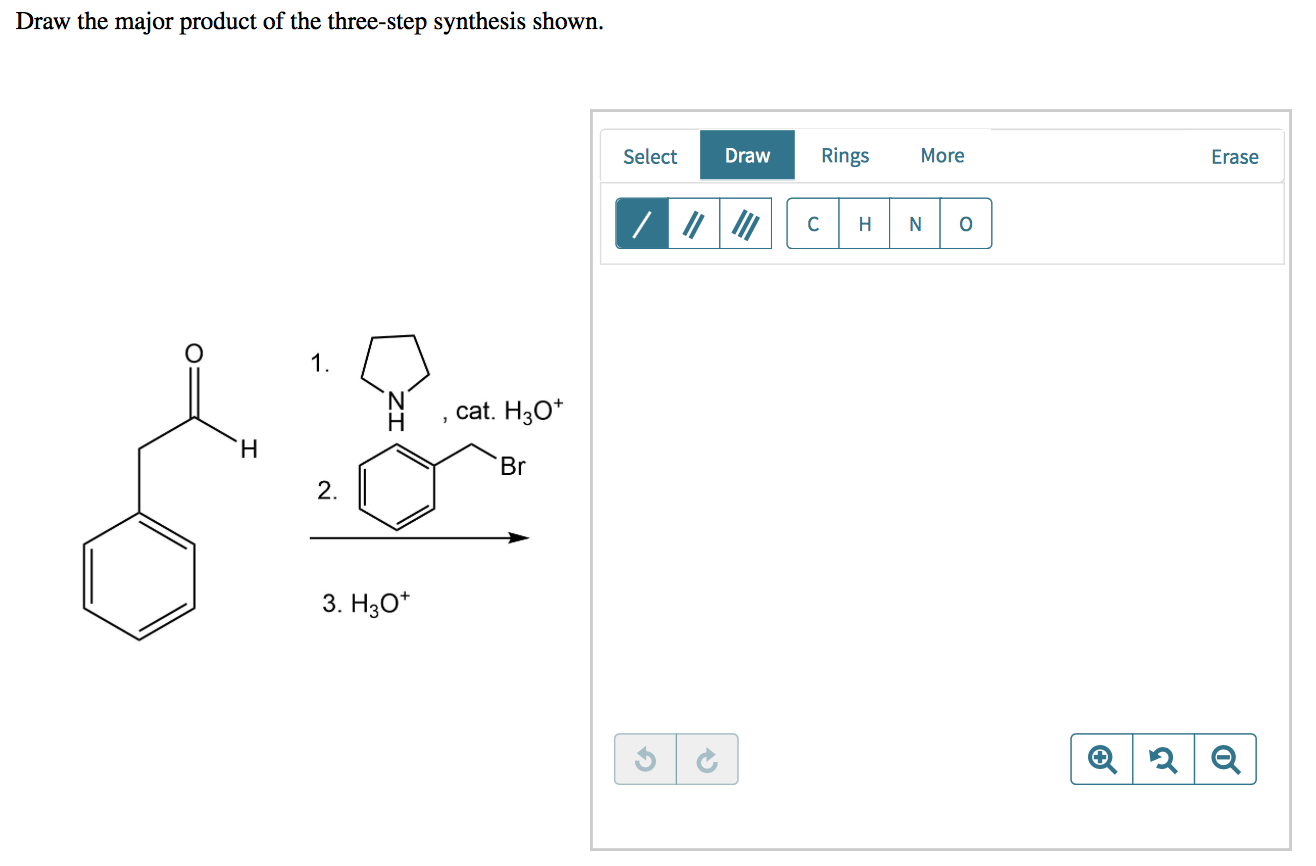
Cat h30* 2 br 3. H30+ h h br 2. The key step in this synthesis is a wolff rearrangement. One step involves dichloroketene while another is a ring expansion reaction. As a start, the benzene ring is drawn for you in each product 1.kmno, naoh,δ hno 2.h30 h2so, febr 02 previous ⓧ give up & view solution check answer next exit hint the first step is an oxidation.
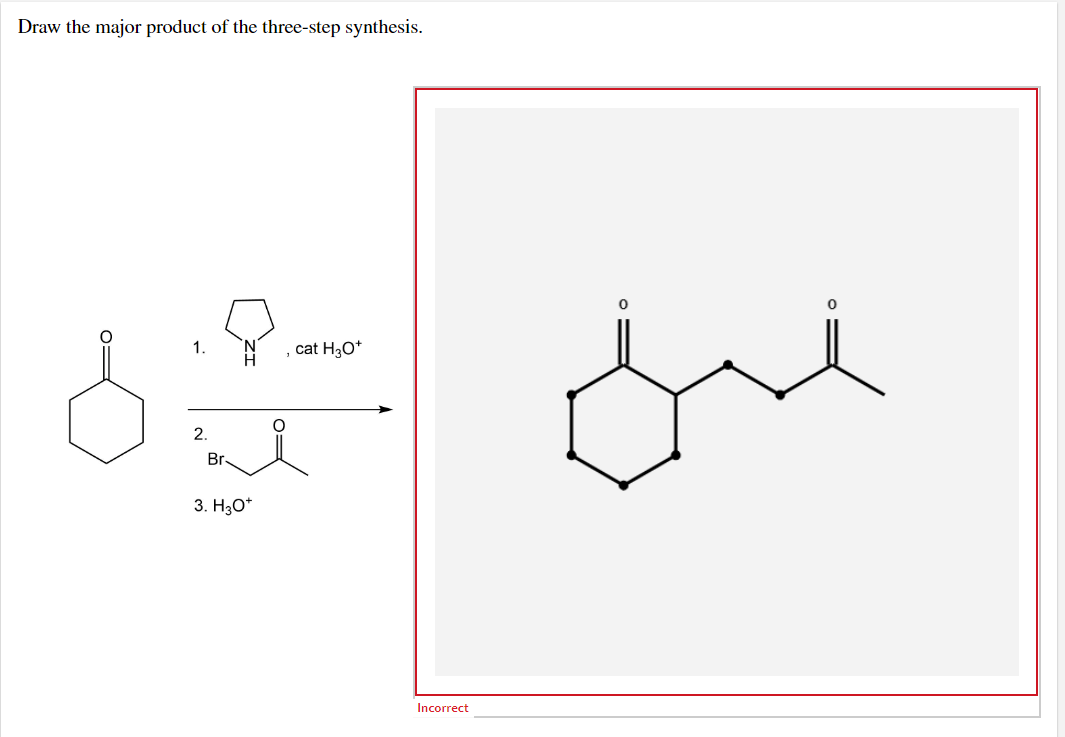
H₂o according to the given question the answer is following as So, the product 1 will have two alcohol groups on the carbon atoms where the double bond was present. The aldhyde group on the final product indicates gentle oxidative cleavage by any of several reaction pathways. Show the formal charges, where applicable. Assuming a very good yield (90%) at each step (this is rarely seen in real projects), a linier synthesis gives 59% overall yield, whereas a convergent synthesis gives 73% overall yield for the same number of steps. Na,cr, 0, product hno, → product 2 h, so br → product 3 febry h, so4 draw product 1 draw product 2 5 0 < question 17 of 21 > attempt 4 incorrect draw product 3 h o incorrect Bromination of benzene in the first step, benzene undergoes bromination in the presence of br2 and febr3 as a catalyst. Select draw rings more с н. As a start, the benzene ring is drawn for you in each product 1.kmno, naoh,δ hno 2.h30 h2so, febr 02 previous ⓧ give up & view solution check answer next exit hint the first step is an oxidation. Draw the organic product of each step in the following synthesis. Na cro bez product hino, → product 2 h,so → product 3 febry h, so draw product 1 draw product 2 on oh x x draw product draw product 2 on oh ŏ o incorre draw product 3 oh š incorrect Web step 1/3 kmno4 is a strong oxidizing agent. Select draw rings more erase c h n o 1. The key step in this synthesis is a wolff rearrangement. One step involves dichloroketene while another is a ring expansion reaction.
H₂O According To The Given Question The Answer Is Following As
Draw the organic product of each step in the following synthesis. Show the formal charges, where applicable. Web step 1/3 kmno4 is a strong oxidizing agent. The aldhyde group on the final product indicates gentle oxidative cleavage by any of several reaction pathways.
(Image) Draw The Major Products For The Following Reaction.
Select draw rings more erase c h n o 1. So, the product 1 will have two alcohol groups on the carbon atoms where the double bond was present. As a start, the benzene ring is drawn for you in each product 1.kmno, naoh,δ hno 2.h30 h2so, febr 02 previous ⓧ give up & view solution check answer next exit hint the first step is an oxidation. The key step in this synthesis is a wolff rearrangement.
However, Without Additional Information, It's Challenging To Draw The Product Structures.
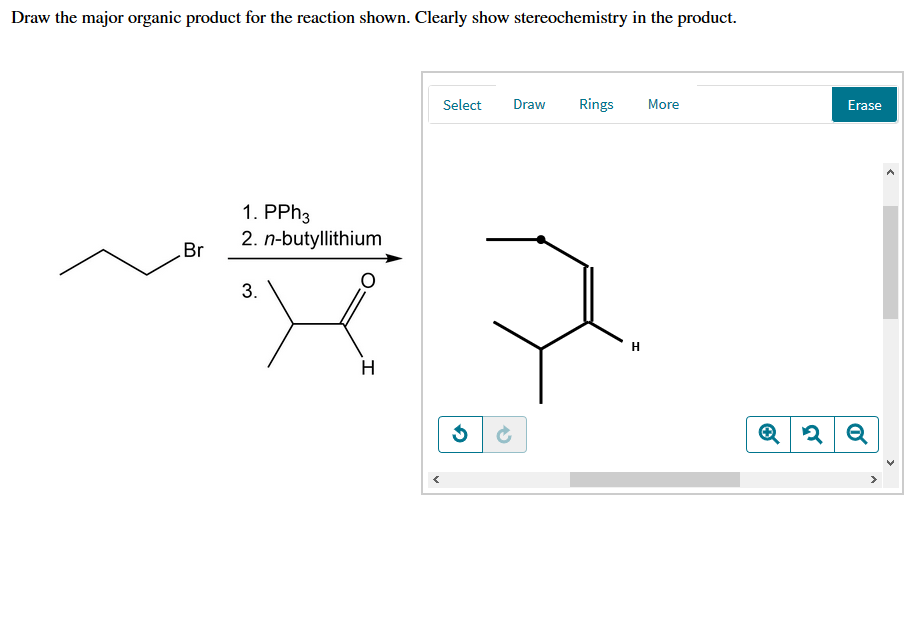
One step involves dichloroketene while another is a ring expansion reaction. Select eras draw rings more 1. Cat h30* 2 br 3. Show the formal charges, where applcable.
Sclect Draw More Erase Coth,O Hio Ring.
Select draw rings more с н. Na,cr, 0, product hno, → product 2 h, so br → product 3 febry h, so4 draw product 1 draw product 2 5 0 < question 17 of 21 > attempt 4 incorrect draw product 3 h o incorrect Draw the major products to the following reactions: Select draw 1 15 cat h,o* 2 br 3.h30* 5